Past announcements will be posted here so you can follow the progress of the trial
Trial Wrap Up (1/26/23)
As many of you know, the CoA-Z in PKAN clinical trial has now come to an end. We were happy that so many in the PKAN community joined the trial, and in fact, the early enthusiasm encouraged us to change our original plan for the study, extending its planned duration from two to three years and expanding the number of people enrolled. Of course, that was before the full impact of the COVID-19 pandemic was known! As the world shut down in 2020 and we saw other clinical trials closed or delayed, we were so pleased that our unusual home-based approach enabled our own CoA-Z trial to continue successfully. Sadly, though, the pandemic did eventually catch up with us: a supply chain problem (compounded by that Texas deep freeze in 2021) led to production delays of CoA-Z, forcing us to make the difficult decision to return to the study’s original design. This did not affect the length of the 6-month “double-blind” portion of the trial, which is key for analyzing the data (this is the time when nobody knows who was taking active CoA-Z and who was taking placebo), and in fact, many participants were able to complete two years in the study as originally planned. Now, we are underway with the important next steps: 1) running multiple blood samples from each participant for biomarker analysis and 2) cleaning up all the data we have collected over the past 2+ years so that it will be ready for statistical analysis. We plan to stay in touch with the PKAN community as we proceed.
We are grateful to each individual with PKAN and their amazing families and caregivers who helped make the trial work. We could not have done it without you!
Progress Update (11/24/21)
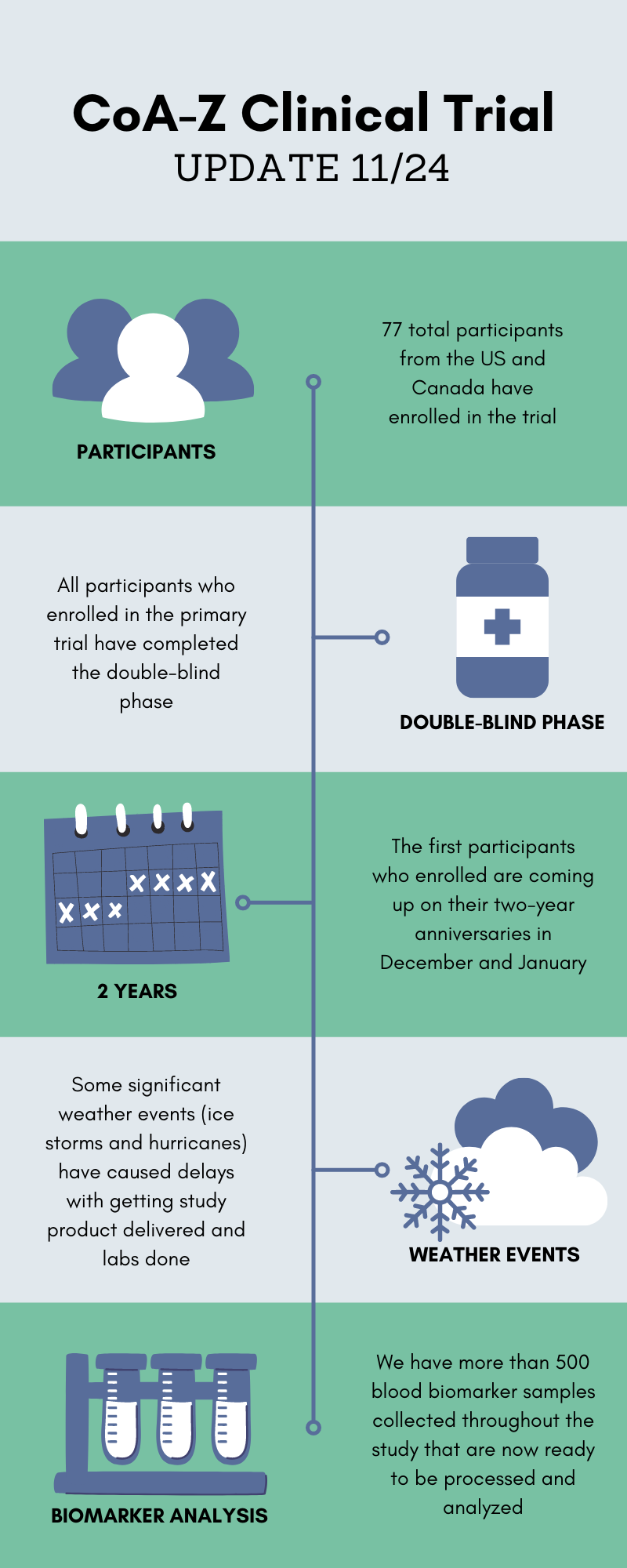
It’s hard to believe it, but the CoA-Z clinical trial started way back in December 2019 when we had no idea how much all our lives were about to be upended by a global pandemic. Many of you have reached out since then to ask for updates and to find out when results will be released. As we approach the two year anniversary, we thought this would be a good opportunity to update the community on how things have been going.
You may recall that the trial was originally planned to be two years in duration for each participant. Since the double-blind phase is now completed and many participants are approaching their key two year study visit, our team has started making plans for data analysis. We know you are all anxious to see the results, but we want to prepare you that this process will take some time. The blood biomarker analysis is one of the most important parts of the study and we have hundreds of samples that must first be processed with great care and consistency.
Overall our team is pleased with how the trial has run for the past two years. We are very grateful for everyone’s commitment to get their study visits done despite their busy lives, unexpected weather events, and even a pandemic. We will share more updates as soon as we can!
Enrollment Started (12/16/19)

The US/Canada CoA-Z clinical trial for PKAN is now enrolling! You can start the process by sending an email to CoAZinPKAN@ohsu.edu and stating that you are interested in the trial. We will try to respond to everyone as soon as we can, but there may be a delay due to the holidays and the number of emails we get.
Launching Soon! (11/22/19)

We are so pleased to finally share that we plan to start enrollment in the US/Canada CoA-Z phase 2 clinical trial in mid-December. We know the PKAN community has waited a long time to hear this news. Please continue to monitor our NBIAcure website and Facebook/Twitter pages for further details as they become available: by keeping you informed online through posting updates, we hope to avoid having our time diverted by a large number of individual telephone and email inquiries. Our team needs to stay focused on the remaining tasks at hand to start the trial! The pace of enrollment will depend on many factors, including our ability to collect information to confirm each person’s diagnosis of PKAN, individually consent each family, manage the logistics of formulating and shipping large volumes of CoA-Z, and coordinate everything with holiday schedules. Please realize that the bulk of enrollment will need to happen during the first quarter of 2020. We are incredibly excited to be at this point and look forward to working with all of you. Please stay tuned for more details!
CoA-Z Paper Publication (11/14/19)
We also want to provide everyone with information about an important new scientific paper authored by our OHSU team in partnership with our Dutch colleagues. The paper was released online in the journal EMBO Molecular Medicine. This peer-reviewed paper helps explain the rationale behind our proposed CoA-Z trial in humans. In addition to providing a link to the paper once it went live, we also developed some Frequently Asked Questions that summarize key points and their relevance for PKAN families. Dr. Sibon’s group in the Netherlands also published a separate paper in the same journal issue that reveals important insights into the biochemical changes in PKAN and related disorders. Together, these two publications provide a solid foundation for launching studies of CoA-Z in people.
You can read both papers and the FAQs at the links below:
CoA-Z Paper FAQs
CoA‐mtACP‐PDH Pathway Paper FAQs
Foundational Study (10/29/19)
In order to prepare for the CoA-Z clinical trial, we are currently completing a small, foundational study of CoA-Z in a small number of adults and children with PKAN. These research participants were the first people to ever be given CoA-Z. This was done under our supervision at OHSU, and they received the compound for a very short predetermined period. The information gained from this short study has helped us refine the dosing plan and schedule of activities for the clinical trial, such as when blood samples will be collected. While this study was not a strict requirement by the FDA, we and our reviewers at the NIH felt it was important to help us confirm that CoA-Z is safe, at least over the short period of this study, and to test some of the processes and parameters we have set for the phase 2 clinical trial before it is officially launched. We are very grateful to the individuals and families who were willing to travel to OHSU for an extended stay in Portland to help us get this critical data. Their contributions will benefit everyone who participates in the upcoming trials.
Stay Tuned (10/27/19)
On this page we will share updates on our work on CoA-Z in preparation for the trial you’ve all been waiting for. We want to share information about the plans for the US and Canadian trial, since we are now close to launch. In addition to the incredible support received through local small grants and your donations to the Spoonbill Foundation, Stichting Lepelaar, and “ZZF”, the Dutch Foundation for rare diseases, all of which have truly kept the trial plans moving forward, our team at OHSU has now also been awarded a large grant from the National Institutes of Health. Specifically, the Eunice Kennedy Shriver National Institute of Child Health and Human Development has awarded us funding to support running the CoA-Z clinical trial over several years in the US and Canada. Spoonbill dollars will continue to be instrumental to fund the ongoing manufacturing and formulation of CoA-Z, database development, and other costs not covered by the grant.