This article and other news can be found in the latest issue of our newsletter which can be downloaded/viewed here: April 2018.
Back in April 2015, the very first beta-testers started participating in our online natural history study, PKANready. These early testers helped us work out the bugs and finalize the design before the full launch of the study later that year. We just started beta-testing our newest study, BPANready, and it’s been three years since the first participant enrolled in PKANready, so we thought this was the perfect time to reflect on what we’ve learned, highlight the value of natural history studies and share some interesting statistics from PKANready.
What is a natural history study?
Natural history studies gather data about the natural progression of a disease. By tracking this data and doing statistical analysis, researchers can learn more about how a disease progresses over time. A natural history study can also identify disease markers (symptoms or measurements) which can be used during a clinical trial to determine if a possible therapeutic is actually changing how that disease would typically progress. Without having a baseline to compare to, it’s incredibly hard to prove that a possible therapeutic is working.
What type of data is gathered?
- Demographics (age, gender, contact information, etc)
- Milestones (smiling, babbling, crawling, talking, walking, etc)
- Surgeries (g-tube, baclofen pump, DBS)
- Medications (current meds and/or changes in meds)
- Imaging (MRIs, ultrasounds, etc)
- Biomarkers
In order to get the best statistical analysis, this data needs to be gathered consistently over time which is why you may be asked the same questions over and over again.
Why are natural history studies important?
In most cases, the FDA requires that a natural history study be completed prior to approval of a clinical trial for a rare disease. That’s why we named these studies PKANready, PLANready and BPANready. We want to be READY to go to clinical trial as soon as a potential therapeutic compound is identified.
Why does it take so long to develop a study?
We want to make sure that we take our time during the design process to ensure that these studies give us data that will actually be helpful moving forward. Our team’s expertise in this group of disorders and our extensive repository allow us to determine the data points that would create the most robust natural history study for each NBIA disorder. We have also learned lessons from the experience of each natural history to apply to the next one. BPANready will be launching soon and the design of that study was directly influenced by what we learned from PLANready, which was influenced by what we learned from PKANready. Each online study has helped us better focus on what data is most helpful and what design or format can get us that data.
We want to thank everyone who tested PKANready/PLANready and are currently testing BPANready because your feedback is invaluable. We also want to thank all the individuals and families who have continued participating in PKANready for the past three years. We understand how difficult it can be to find time in your busy lives to complete our questionnaires, but we can confirm that you are doing an invaluable service to the entire NBIA community.
What have we gained from these studies?
The data gathered over the past three years through PKANready directly impacted the FDA’s positive response to CoA-Z and our ability to progress so quickly to clinical trial. It probably doesn’t feel quick for all of you, but it absolutely is in comparison to how clinical trials usually progress (especially for rare diseases).
If you want to learn more about PKANready or PLANready, please visit one of the links below. We will make an announcement on our website and social media accounts as soon as BPANready launches.
PKANready Statistics
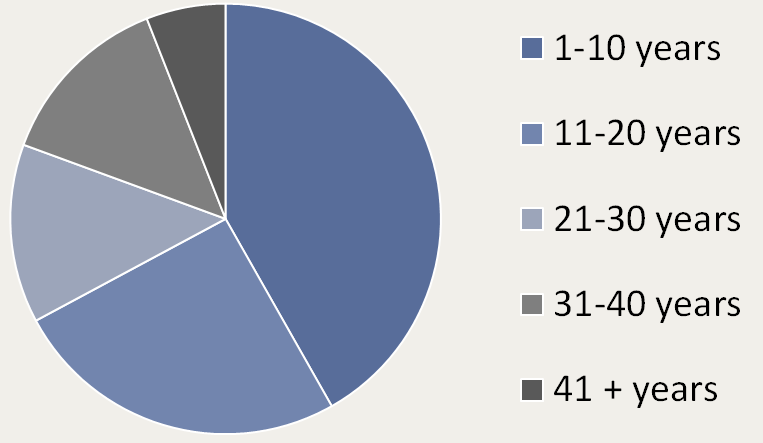
Participants with both classic and atypical PKAN can enroll in PKANready so this study includes individuals ranging from 3 years old to 60 years old. Due to the increased availability of whole exome sequencing, we’ve seen a dramatic increase in the number of individuals diagnosed before age 10.
Age ranges of PKANready participants
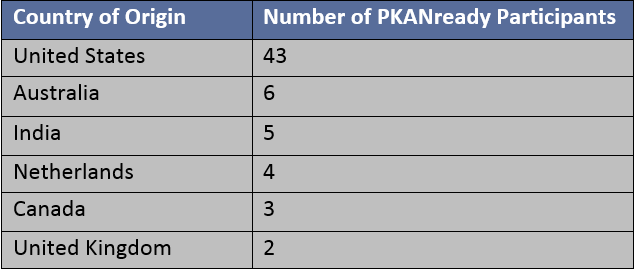
Number of participants from each country