If you or a loved one has recently been diagnosed with PLAN/aNAD, we hope our website can help answer some of your questions and guide you to the most appropriate resources. We encourage you to call our team to speak to a genetic counselor who can help you navigate this diagnosis. Please email us at info@nbiacure.org and we will set up a phone call with you.
PLAN (PLA2G6-associated neurodegeneration) is a NBIA (neurodegeneration with brain iron accumulation) disorder. Based on an individual’s age of onset and symptoms, they may be classified as having one of three types of PLAN: INAD, aNAD or PLA2G6-related dystonia-parkinsonism. However, as we learn more about PLAN, we have found that some people fall between these 3 categories. In other words, there is a broad spectrum of symptoms.
SYMPTOMS
The symptoms of aNAD (atypical neuroaxonal dystrophy) are more variable than those of INAD. Individuals typically start showing symptoms in early childhood, usually by 4 years of age.
Common symptoms include:
Speech delay and autistic features
- May be the first evidence of early disease
Psychomotor (mental and physical ability) regression
- Development may seem to slow, fewer skills are gained
- Loss of previously acquired milestones may eventually occur
- Gait instability (unstable walking) or ataxia (lack of muscle control)
- Often the first symptom that is noticed
Dystonia (involuntarily muscle contraction and spasms) and dysarthria (poor articulation or slurring of speech)
- Seen more in aNAD than in INAD
- Leads to speech problems that can make it difficult to be understood by acquaintances and others in the community. Usually people close to the individual with aNAD have learned to better understand their speech.
Spastic tetraparesis (muscle tightness and weakness in both arms and legs)
- Seen later in the disease course
- Hypperflexia (overactive reflexes) develops first
- Areflexia (absent reflexes) develops later
Joint contractures (reduced joint mobility)
- Side effect of prolonged tightness and stiffness in muscles
Eye features
- Nystagmus (rapid uncontrolled eye movements)
- Optic atrophy (deterioration of the nerve that connects the eye to the brain)
Seizures (rare)
- Occurs later in the disease
CAUSE/GENETICS
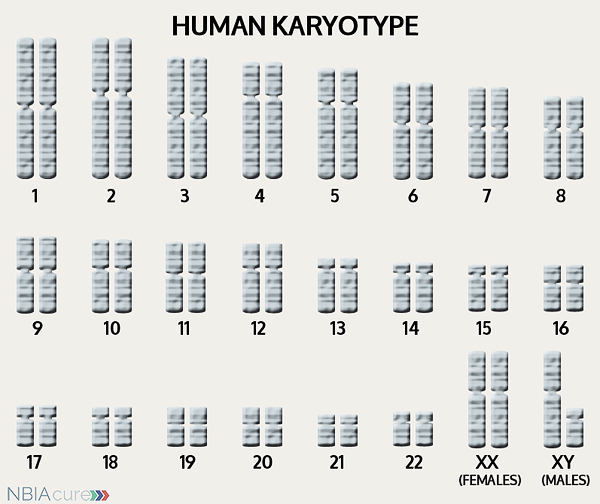 The human body is made up of millions of cells. Inside every cell there is a structure called DNA, which is like an instruction book. DNA contains detailed steps about how all the parts of the body are put together and how they work. However, DNA contains too much information to fit into a single “book,” so it is packaged into multiple volumes called chromosomes. Humans typically have 46 total chromosomes that are organized in 23 pairs. There are two copies of each chromosome because we receive one set of 23 chromosomes from our biological mother and the other set of 23 from our biological father. Chromosomes 1-22 are called autosomes and the last pair is called the sex chromosomes because they determine a person’s gender. Females have two X chromosomes and males have one X and one Y.
The human body is made up of millions of cells. Inside every cell there is a structure called DNA, which is like an instruction book. DNA contains detailed steps about how all the parts of the body are put together and how they work. However, DNA contains too much information to fit into a single “book,” so it is packaged into multiple volumes called chromosomes. Humans typically have 46 total chromosomes that are organized in 23 pairs. There are two copies of each chromosome because we receive one set of 23 chromosomes from our biological mother and the other set of 23 from our biological father. Chromosomes 1-22 are called autosomes and the last pair is called the sex chromosomes because they determine a person’s gender. Females have two X chromosomes and males have one X and one Y.
If DNA is the body’s instruction book and it is stored in multiple volumes (called chromosomes), then genes would be the individual chapters of those books. Genes are small pieces of DNA that regulate certain parts or functions of the body. Sometimes multiple genes (or chapters) are needed to control one function. Other times, just one gene (or chapter) can influence multiple functions. Since there are two copies of each chromosome, there are also two copies of each gene. In some gene pairs, both copies need to be expressed (or turned on) in order for them to do their job correctly. For other genes pairs, only one copy needs to be expressed.
When a single cell in the human body divides and replicates, its DNA is also replicated. This replication process is usually very accurate but sometimes the body can make a mistake and create a “typo” (or mutation). Just like a typo in a book, a mutation in the DNA can be unnoticeable, harmless, or serious. A mutation with serious consequences can result in a part of the body not developing correctly or a particular function not working properly.
In the case of NBIA disorders, changes in certain genes cause a person to develop their particular type of NBIA. Changes in these NBIA genes lead to the groups of symptoms we observe, although we do not yet understand how the changed genes cause many of these findings. PLA2G6 is the only gene known to cause all the types of PLAN. PLA2G6’s main job is to tell the body’s cells how to make an enzyme called A2 phospholipase that breaks down phospholipids. When a change the PLA2G6 gene impairs the function of this enzyme, the cells’ membrane maintenance is disrupted and may lead to the development of spheroid bodies in the nerve axons. It is not yet clear to us how the decrease in A2 phospholipase eventually leads to iron accumulation in the brain.
As mentioned earlier, humans have a total of 23 pairs of chromosomes. Half of these chromosomes are passed down (or inherited) from the biological mother and half from the biological father. The way in which a gene carrying a change is passed down from parents to child varies from gene to gene. The PLA2G6 gene that is altered in those with aNAD is inherited in an autosomal recessive manner.
“Autosomal” refers to the fact that the PLA2G6 gene is located on chromosome 22, which is one of the autosomes (chromosome pairs 1-22). Since the sex chromosomes are not involved, males and females are equally likely to inherit the changed gene. “Recessive” refers to the fact that a gene change must be present in both copies of the PLA2G6 gene for a person to have aNAD. If an individual has only one PLA2G6 gene change, then they are called a “carrier” for aNAD. Carriers do not have health problems related to that gene change and often do not know they carry a recessive gene change. However, if two aNAD carriers have a child together, then there is a 25% chance that they will both pass on their recessive PLA2G6 gene changes and have a child with aNAD.
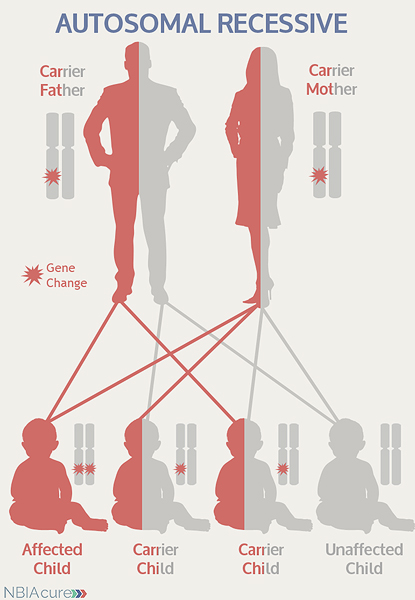 As seen in the image to the left, in a pregnancy between two aNAD carriers:
As seen in the image to the left, in a pregnancy between two aNAD carriers:
- There is a 25% chance of the child having aNAD
- There is a 50% chance that the child will be a carrier like his/her parents
- There is a 25% chance that the child will not have aNAD or be a carrier
DIAGNOSIS & TESTING
An MRI of the brain and an ophthalmologic exam are key tests used to establish the clinical symptoms of aNAD. MRI stands for magnetic resonance imaging. An MRI produces a picture of the body that is created using a magnetic field and a computer. The technology used in an MRI is different from that of an x-ray. MRI is painless and is even considered safe to do during pregnancy. Sometimes an MRI is done of the whole body, but more often, a doctor will order an MRI of one particular part of the body.
An MRI is often ordered as part of the work-up of children with aNAD symptoms. However, it may be normal, especially early in disease. A T2 sequence is the preferred type of MRI because it is highly sensitive to the detection of brain iron.
MRI findings for aNAD include:
- Cerebellar changes
- Cerebellar atrophy (degeneration of the cerebellum)
- Cerebellar hyperintensities (brightness)
- Indicates iron accumulation
- Not all individuals with aNAD have iron accumulation
Before genetic testing was available, other tests were used to try to confirm a diagnosis of aNAD:
- Biopsy of one or more of the following tissues to look for axonal spheroids in the nerves:
- Conjunctiva (outer layer of the eye)
- Skin
- Rectum
- Muscle
- Other peripheral nerve
Now, a diagnosis of aNAD is confirmed through genetic testing of the PLA2G6 gene to find two gene changes. At least one PLA2G6 gene change is found through DNA sequence analysis in ~85% of individuals.
If no gene change or only one gene change is found through sequence analysis of the PLA2G6 gene, then genetic testing may proceed to deletion/duplication analysis. Among individuals who go forward with deletion/duplication analysis, a gene change may be found in ~12.5% of cases.
Sometimes an individual with the signs and symptoms of aNAD will have only one or even no PLA2G6 gene changes identified. This can happen because the genetic testing is not perfect and has certain limitations. It does not mean the person does not have aNAD; it may just mean we do not yet have the technology to find the hidden gene change. In these cases it becomes very important to have doctors experienced with aNAD review the MRI and the person’s symptoms very carefully to be as sure as possible of the diagnosis.
MANAGEMENT
 There is no standard treatment for aNAD. Diagnosed individuals are managed by a team of medical professionals that recommends treatments based on current symptoms.
There is no standard treatment for aNAD. Diagnosed individuals are managed by a team of medical professionals that recommends treatments based on current symptoms.
After diagnosis, the following evaluations are recommended to determine the extent of disease:
- Ophthalmologic (eye) exam to look for optic atrophy
- EEG to find any unrecognized seizure activity
- Genetics consultation
Long-term management for aNAD can include:
- Medication for spasticity, dystonia and/or seizures
- Trial of oral or intrathecal baclofen for those with significant dystonia
- Addition of gastric feeding tube or tracheostomy (if needed)
PROGRESSION
Individuals with aNAD are fairly stable during their early childhood, but start declining between ages 7 and 12.
aNAD cases are rare, so the average life span is not known. However, the life span is expected to be longer than that of classic INAD because aNAD symptoms develop at a later age.
RESEARCH
You can currently enroll in (or enroll your child in) a natural history study called PLANready. The purpose of this study is to help us better understand the progression of PLAN/aNAD and identify disease markers that can be used in future clinical trials. This study can be done completely from home and involves entering information every three-six months. You can learn more about this study and enroll here: PLANREADY
Copyright © 2014 by NBIAcure.org. All rights reserved.