 A transgenic mouse is a mouse that has artificially-added genetic material in some or all of its cells. The extra genetic material or “foreign DNA” can come from any source or species. This process is used to make mouse models of human diseases.
A transgenic mouse is a mouse that has artificially-added genetic material in some or all of its cells. The extra genetic material or “foreign DNA” can come from any source or species. This process is used to make mouse models of human diseases.
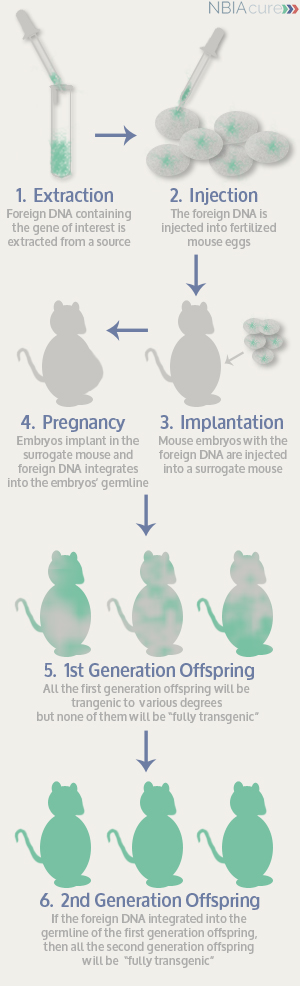
A transgenic mouse is created by first injecting foreign DNA into a fertilized mouse egg. The fertilized egg is inserted back into the female mouse the egg came from or into a different surrogate mouse that will carry the developing egg. The foreign DNA integrates into the mouse embryo’s own DNA after the cells in the egg have divided a few times. However, in most cases the foreign DNA does not integrate into all the cells of the mouse embryo, which makes it only partially “transgenic.” At this point, all we can do is hope that the foreign DNA has integrated into the mouse embryo’s germline (the cells that will go on to form its sperm or eggs). If the foreign DNA is in the mouse embryo’s eggs or sperm, then when that mouse has pups, some of them will have the foreign DNA in all the cells of the body and they will be fully transgenic.
Research involving transgenic mice can take a long time to conduct and may need to be restarted multiple times. During just the process of creating the mice, many things can go wrong:
- The foreign DNA may not integrate into the germline and the mouse’s offspring are not fully transgenic
- The foreign DNA may integrate incorrectly and cause harmful side effects to the mouse
- The mouse with the foreign DNA may not have enough pups or healthy pups
These are just some of the reasons why we must go through many trials and errors before we even have the correct transgenic mice to start using for our research.
The two main types of mice used by our research group for NBIA disorders are “knock-out” and “knock-in” mice. These mice are used to study the effects NBIA disorders have on different organs, the progression of symptoms and to test potential new treatments.
PKAN Knock-Out Mice
When we first started studying NBIA disorders, we worked with collaborators at UCSF to develop knock-out mice. In order to simulate the PKAN patients whose natural genetic changes “turned off” their PANK2 genes, the PKAN mice had their PANK2 genes artificially turned off or “knocked out”.
Unfortunately, these mice didn’t turn out to be the best PKAN test animals because they do not have the neurological symptoms or brain iron accumulation seen in humans. Although they do have some features of PKAN and have been useful for many experiments, with the development of new mouse technology we thought we could make a better mouse model.
PKAN Knock-In Mice
After the knock-out mice didn’t work out the way we had planned, we changed our method and created knock-in mice instead. The knock-in mice were developed by a company called OzGene, and the project is funded by the NBIA Disorders Association (NBIADA). We and the NBIADA worked with OzGene to develop mice that have a specific gene change added into the exact location we are interested in. We hope this will better simulate how PKAN affects humans. Using newer technologies, this mouse has several bells and whistles that weren’t available before. For example, we can “turn on” the genetic change only in specific tissues, such as the liver or the brain, to see what kind of changes occur as a result. The NBIADA funded the development of this mouse and can provide it to PKAN investigators worldwide.
Other NBIA Transgenic Mice
Several other mouse models of NBIA exist. Some have occurred naturally, while others were engineered. Although there are many pitfalls to using mouse models, they remain an invaluable tool in understanding NBIA and testing potential treatments.
Copyright © 2014 by NBIAcure.org. All rights reserved.